Deciphering the molecular mechanisms of pathology and disease variability of sporadic and familiar Alzheimer’s.
For the last 10 years we have characterized the neuropathology of the brains collected from the largest population in the world sharing a single common mutation (E280A) in PSEN1. We have learned that there is wide variability in the pathological presentation of Alzheimer’s in these brains, often associated with modifications in its clinical presentation. Furthermore, we have identified specific cellular and molecular features for this mutation following and translational approach, from pathological features identified in the brains to cellular and animal models. We expect that all the knowledge obtained from these cases will help to a better understanding of both familial and sporadic Alzheimer’s presentations in view of the development of successful therapies for the disease.
In-depth phenotypic characterization of familial and sporadic Alzheimer’s neuropathology
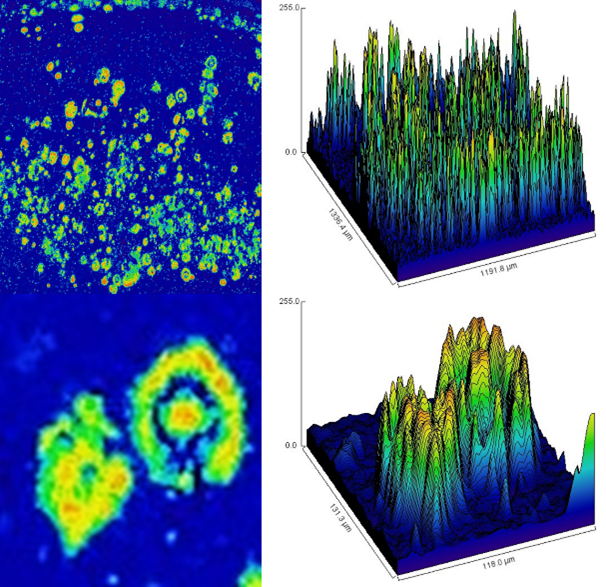
Our colleagues in Colombia have collected close to 300 Alzheimer’s disease brains so far. 130 brains donated by PSEN1 E280A carriers and more than 150 from sporadic Alzheimer’s patients. Using classic neuropathological methods, we are studying and diagnosing all those brains to assess variability in the distribution and pattern of presentation of Amyloid beta (Aβ) plaques and hyperphosphorylated tangles deposition. We are now using high throughput methods, molecular and neuropathological, to assess phenotypic variability and its possible relation with clinical presentation subtypes together with colleagues in Colombia, Brazil and United States.
Collaborators:
Prof. Francisco Lopera
Funding:
- DFG – Identifizierung von Hirnzelltyps-pezifischen Genregulationsnetzwerken bei Patienten mit familiärer Alzheimer-Krankheit PSEN1 E280A. 458854216
- Alzheimer’s Association – AI assisted neuropathological assessment of sporadic Alzheimer’s cases 23AARGD-1030514
- Digital Neuropathology – Project GADIR
Identification of mechanisms of resistance and disease modifiers in familial Alzheimer’s disease
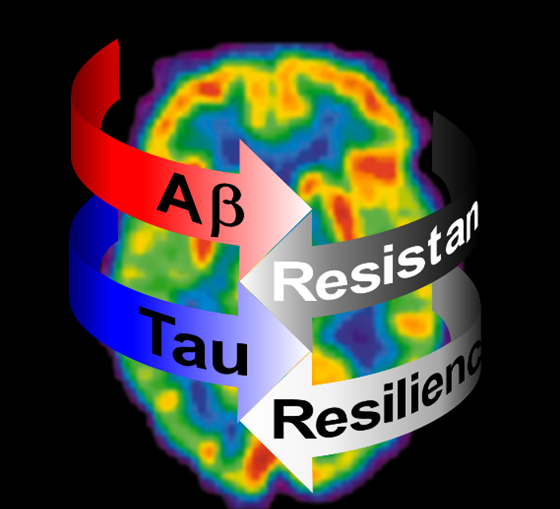
In some PSEN1 E280A cases we have detected a correlation between decreased cortical Tau pathology and delayed dementia onset, sometimes more than 30 years. These special cases have provided clues for molecular mechanisms of resistance against the pathophysiology of the disease. However, there are even more rare cases that although presenting with full-fledged Alzheimer’s pathology, also present with the same degree of delayed disease onset. These cases offer a clue about mechanisms of resilience against Alzheimer’s disease and neurodegeneration in general. A deep understanding of mechanisms of resistance and resilience against Alzheimer’s disease is a necessary step for the development of successful therapies. Furthermore, age at disease onset is not the only phenotypic feature indicating risk or protection. We are also exploring genetic modifiers of cognitive decline.
Collaborators:
Prof. Francisco Lopera
Dr. Joseph F. Arboleda-Velásquez
Funding:
- Good Ventures – Mechanisms associated with resilience to Alzheimer’s disease.
- Bürgerstiftung Hannover / Wilhelm-Emanuel-Zach-Stiftung – Identifizierung und Validierung von protektiven Mechanismen in Gehirnen von Patienten, welche vor der Alzheimer-Krankheit geschützt sind. Projekt 2023-119.
- Bürgerstiftung Hannover / Wilhelm-Emanuel-Zach-Stiftung – Identifizierung und Validierung von protektiven Mechanismen in Gehirnen von Patienten, welche vor der Alzheimer-Krankheit geschützt sind. Projekt 2025-89.
Small vascular pathology in familial Alzheimer’s disease
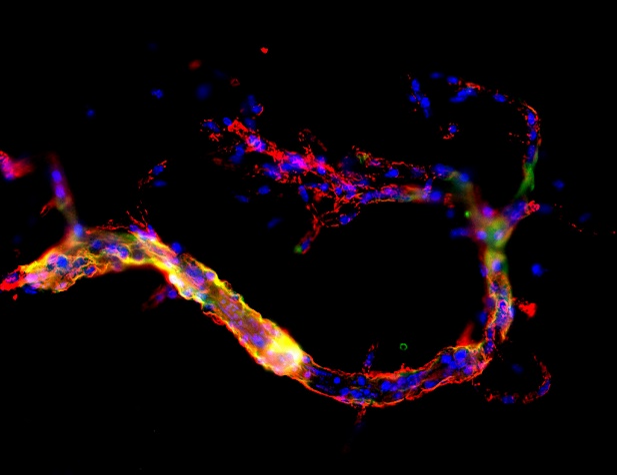
A high number of sporadic Alzheimer’s disease cases present with vascular pathology together with the more common Alzheimer’s neuropathological hallmarks. For long these vascular changes have been attributed to Ab accumulation in blood vessels. We have identified that in familial Alzheimer’s cases there are other vascular changes not directly associated with Ab accumulation, and that could be related with specific cellular functions of Presenilin-1. We are currently identifying the molecular pathways associated to small vessel pathology in PSEN1 mutants and their possible impact in clinical phenotypes.
Collaborators:
Prof. Francisco Lopera
Dr. Joseph F. Arboleda-Velasquez
Dr. Yakeel T. Quiroz
Funding:
- NIH – Charting vascular contributions to white matter disease in familial Alzheimer’s disease and CADASIL. RF1NS110048-01S1
Mechanisms of stress and cellular vulnerability in PSEN1 mutants
From the findings identified in patient’s brain tissue we have learned that PSEN1 mutant cells are more vulnerable to cellular stress via mechanisms independent of Aβ accumulation or Aβ altered production. Instead, gamma secretase disfunction has an impact in cellular homeostasis rendering cells more vulnerable to stress. We have confirmed these findings in various cellular models, and we are currently assessing the degree of vulnerability in specific cellular populations in the brain and their possible impact in neurodegeneration. These findings can be relevant for unraveling Alzheimer’s the early onset and high severity typically found in familial Alzheimer’s disease patients. For this project we are leveraging on single nuclei and spatial transcriptomics to identify vulnerable cell types.
Funding:
- DFG – Identifizierung von Hirnzelltyps-pezifischen Genregulationsnetzwerken bei Patienten mit familiärer Alzheimer-Krankheit PSEN1 E280A. 458854216
- Bürgerstiftung Hannover / Wilhelm-Emanuel-Zach-Stiftung – Identifizierung und Validierung von protektiven Mechanismen in Gehirnen von Patienten, welche vor der Alzheimer-Krankheit geschützt sind. Projekt 2023-119.
- Bürgerstiftung Hannover / Wilhelm-Emanuel-Zach-Stiftung – Identifizierung und Validierung von protektiven Mechanismen in Gehirnen von Patienten, welche vor der Alzheimer-Krankheit geschützt sind. Projekt 2025-89.